The fiber optic and cable industry is very familiar with quartz glass. The technology of conventional optical fiber is very mature, with a very low cost, excellent optical performance, and the mechanical performance is also very good. Less contact with the infrared (IR) transparent glass. In fact, the glass family is very large, such as fluoride and sulfide glass (non-oxide) also has good optical properties. There are also many application scenarios suitable for infrared, such as: infrared imaging, medical, astronomy and biological application sensors.
The advantages and disadvantages of silica glass: silica (SiO2) and other oxide glass, unique and excellent performance dominates the optical field. From ultraviolet (UV) to visible (visible) to near-infrared (NIR), red is the sensitive area of our retina. Silica dioxide is an excellent glass forming agent, the mechanical strength of the material is very high, but also better resistance to crystallization and corrosion.

Quartz glass is actually sand SiO2 glass has an inherent drawback: wavelength more than 3 μ m opaque. The transparency limitation is due to the high vibrational mode (high vibration mode) of the Si-O bonds. In order to develop optical devices that can transmit wavelengths of more than 3 μ m, we should look for new components with weaker chemical composition (weaker chemical bond) and composed of heavier atoms (heavier atoms). Fluoride (fluoride) and sulphide glass have good advantages.

What are the basic criteria for obtaining glassy materials? It must have polymerization properties (polymeric character); bond angles (bond angle) are easily changed to melt into a viscous liquid (viscous liquid); and macromolecules are formed by covalent bonds (covalent bonding). In general, polymer materials may also be large anions (ion). The negative charge (negative charge) is compensated by some large cations (large cations) distributed in the viscous liquid. These large cations are called glass modifiers (modifier).
How is the glass made up of atoms? Glass is a "disordered state." Take silica, which exists in many forms, from calcite to quartz. Different states are formed only by the different values of the Si-O-Si angles, generally formed by angle-sharing SiO4 tetrahedra. So when the silica melts, the atoms don't choose a certain direction and remain disordered in the liquid state. This is almost the same when using materials such as fluoride glass or sulfur-element glass. When the liquid is cooled at a certain temperature, the liquid transforms into a crystalline solid. This is the case for most liquids.
Thus, some of these liquids refuse to follow this law of thermodynamics. Instead, they become more and more sticky until the viscosity becomes infinite (viscosity becomes infinite). The solid obtained is a frozen liquid (frozen liquid), which is the glass. Depending on the glass composition, the cooling rate generally requires fast, fast enough, enough to avoid any arrangement of atoms that will nucleate the microcrystals. This process is the core of glass forming! This temperature quenching process (temperature quenching procedure) is empirical, which is definitely one of the secrets of glass manufacturers.

After annealing, the glass is frozen liquid or liquid with infinite viscosity.Showed that the bonds are continuous and uniform. As a transmittance material: in ultraviolet light, the intrinsic absorption (intrinsic absorption) caused by the interaction with bonded electrons, in the interaction between the infrared region and the matrix (interactions with the phonon of the matrix), there is no reason to interrupt the light propagation.
Glass has some unique properties compared to other solids. The glass can be heated to a temperature region called the glass transition temperature (Tg). Above Tg, the glass remembers that it is liquid and later becomes a plastic solid, and its viscosity changes rapidly with temperature. The control of viscosity is very critical, and the control of glass Tg can realize the complex processes such as die pressing and optical fiber preparation. Because glass is an unbalanced solid, the forming process of glass may have the risk of nanocrystal nuclei, which will be separated from the glass matrix. Partial crystallization may have a large influence on the propagation of optical fibers. This disordered unbalanced solid is easily transformed into ordered crystals depending on the energy distribution of the glass.
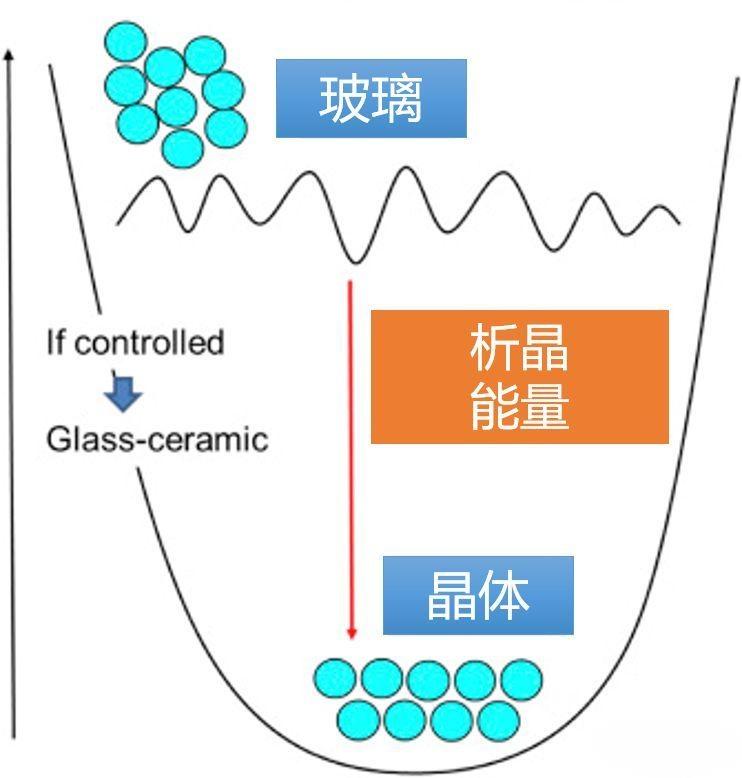
The crystallization phenomenon can be confirmed by detecting the crystallization temperature by differential scanning calorimetry (differential scanning calorimetry) (distinguishing that Tx is the clean temperature and Tg is the glassy temperature). The difference between Tx-Tg is the standard for assessing the glass shape. Try to control and avoid the generation of nanocrystal, because it will scatter light in IR, become an obstacle to IR fiber transmission.